
Pallavi Tiwari, PhD received a $3.4 million, multi-institutional grant from the National Institutes of Health/National Cancer Institute for the project “Radiomic spatial maps for identifying viable tumor extent on multi-parametric MRI for Glioblastoma.” This novel project will be the first to utilize artificial intelligence (AI) to detect hard-to-see glioblastoma tumor edges during a clinical trial.
Glioblastoma is an aggressive cancer with an average 15-month life expectancy once diagnosed. Surgery is typically the main treatment option due to its’ fast-acting nature; however, the tumors often reach microscopic niches in the brain tissue that are not visible during surgery. As a result, most surgical procedures don’t completely remove the tumor and it continues to spread throughout the brain.
Microscopic niches pose another problem – existing methods to determine tumor edges, like biopsies, may miss these areas and lead to inaccurate results. These methods are not routinely done as part of a clinical practice due to the expense and need for patients to travel for the procedure.
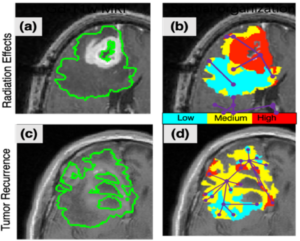
The project’s overarching goal is to use AI to accurately predict and map out where the tumor is, resulting in complete removal during surgery. Dr. Tiwari explained how the research group will evaluate the effectiveness of AI in determining where the tumor ends and where healthy brain tissue begins in the clinical trial phase.
“We use spatial radiomaps (i.e. “virtual biopsy maps”), which act as a sort of GPS map. We take a simple MRI scan of the patient’s brain, and then apply the AI analysis to create the radiomap. From there, we look to see where the map says the tumor is, and we perform a biopsy to check for accuracy and see if that tissue is cancerous.”
This is a multi-institutional endeavor, with Dr. Tiwari serving as the primary investigator (PI) along with Manmeet Ahluwalia, MD from the Miami Cancer Institute who will lead the clinical trial. Researchers from the Cleveland Clinic Lerner College of Medicine and the H. Lee Moffitt Cancer Center & Research Institute are also contributing to the project.
Multi-institutional research projects can be challenging, especially when sharing patient data is concerned, but Dr. Tiwari emphasized that the collaborative nature is one of the key strengths of this project.

“When training AI models, diversity of data is key; and the multi-institutional approach provides an advantage. Having a diverse set of studies from multiple sources helps ensure that accurate results from the AI analysis are reproducible from one case to another, across institutions.”
Dr. Tiwari highlighted that having the AI analysis use the imaging information, with corresponding pathology, and genomic information, to create biologically inspired image-features that drive the analysis is a cornerstone of the project.
Although in the early stages, the project has exciting potential to improve survival rates of glioblastoma patients, and down the road, patients with other types of cancer with similarly hard to detect tumor edges. One of the biggest advantages is that the AI analysis is done on routinely acquired MRI images. With no special equipment or advanced imaging required, healthcare providers can keep costs for patients down.
MRI imaging is widely available and part of standard-of-care, so as patients get scanned, they can have the AI analysis run, and within 24 hours, have their tumor radiomap sent to the surgeon. Potentially, a patient could undergo surgery in as little as two days from when they are scanned. When dealing with an aggressive cancer like glioblastoma, this short turnaround time could have a profound impact on patient survival rates.