Led by Amy Fowler, MD, PhD, the Hormone Receptor Molecular Imaging and Biology Lab’s research focuses on advancing the use of molecular imaging to better understand the biology of breast cancer including its response to targeted drug therapies and early identification of drug resistance. Predictive and pharmacodynamic molecular imaging biomarkers may help physicians choose the best treatment tailored to each patient based on their specific tumor signaling characteristics and ultimately improve survival. Specifically, the lab is interested in quantitative imaging of steroid hormone receptors (estrogen and progesterone receptor) and their associated signaling pathways since the majority of breast cancer deaths occur in patients with metastatic estrogen receptor alpha (ER) positive disease.
The lab’s experimental approaches span the translational continuum from preclinical (cell-based assays and tumor xenograft models) to clinical studies. Currently the lab focuses on using 18F-labeled radiopharmaceuticals and multimodality imaging with positron emission tomography (PET) and computed tomography (CT) or magnetic resonance imaging (MRI). Additionally, optical imaging (fluorescence and bioluminescence) is an important tool used for the preclinical work.
Lab News
PET/MR Imaging: New Possibilities for Breast Cancer Management
February 9, 2023Amy Fowler Awarded R01 Grant
October 13, 2022Amy Fowler and Colleagues Publish in Radiology: Imaging Cancer
February 9, 2021
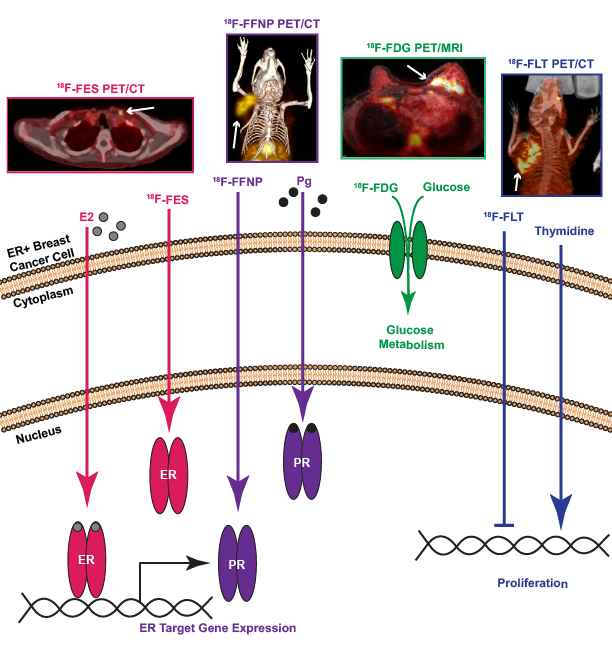
The Hormone Receptor Molecular Imaging and Biology Lab focuses on molecular imaging biomarkers of the estrogen signaling pathway in estrogen receptor (ER) positive breast cancer. Positron emission tomography (PET) imaging using a radiolabeled estrogen, 18F-fluoroestradiol (FES), is a read-out of the ligand binding functionality of ER.
An axial fused PET/computed tomography (CT) image demonstrates FES uptake in a left supraclavicular lymph node in a patient with metastatic ER+ breast cancer (pink, arrows 1, 2, from left). Progesterone receptor (PR) is a classic estrogen-regulated ER target gene. PET imaging with a radiolabeled progestin analog, 18F-fluorofuranylnorprogesterone (FFNP) is an endogenous read-out of ER functional transcriptional activity.
A maximum intensity projection fused PET/CT image demonstrates FFNP uptake in a PR+ mammary carcinoma cell line grown as a murine xenograft located in the right thoracic mammary fat pad (purple, arrows 3,4 from left). Estrogen signaling also increases glucose uptake through genomic and non-genomic mechanisms. PET imaging with a radiolabeled glucose analog, 18F-fluorodeoxyglucose (FDG), reflects tumor glucose metabolism in vivo.
An axial fused PET/magnetic resonance imaging (MRI) image demonstrates avid FDG uptake in a patient with inflammatory left breast cancer and abnormal left internal mammary lymph node (green, merging arrows). Estrogen signaling culminates in increased tumor cell proliferation which can be measured by PET imaging with a radiolabeled thymidine analog, 18F-fluorothymidine (FLT).
A maximum intensity projection fused PET/CT image demonstrates FLT uptake in a tumor xenograft located in the right thoracic mammary fat pad (blue, right 2 arrows).
Current Funding and Grants
Training Programs and Other Affiliations
Training Programs
The Hormone Receptor Molecular Imaging and Biology Lab consists of scientists, post-docs, graduate students, and undergraduates working in a collaborative, multidisciplinary environment focused on translational breast cancer imaging research. There are occasionally medical students, residents, and fellows involved with the lab’s clinical research projects.
Lab Members' Expertise
 |
 |
 |
||
|
Cell and Molecular Biology |
Cancer Biology |
Tumor Genomics |
Radionuclide Imaging |
Clinical Research |
The lab welcomes individuals with experience in, or wishing to gain experience in:
- The design and performance of cancer biology/molecular imaging experiments using in vitro and in vivo models of breast cancer
- Design and implementation of clinical trials of molecular imaging agents for breast cancer
- Writing/editing manuscripts and grants
Open positions will be posted here when available.
Principal Investigator (PI)

Amy Fowler, MD, PhD
Publications at NCBI My Bibliography
Education
Undergraduate: South Dakota State University (Brookings, SD)
Graduate School: University of Wisconsin–Madison, Cellular and Molecular Biology Program
Medical School: University of Wisconsin School of Medicine and Public Health
Internship: Gundersen Lutheran Medical Center (La Crosse, WI)
Residency: Mallinckrodt Institute of Radiology, Washington University (St. Louis, MO)
Fellowship: Mallinckrodt Institute of Radiology, Washington University (St. Louis, MO)
Current Lab Members

Mary Pat Berry, BA, MA
Breast Cancer Research Advocate

Blue-Leaf Cordes, MD, PhD
Scientist III

Mary Ozers, PhD
Scientist III

Kelley Salem, PhD
Scientist I

Natasha Solodin, BS
Scientist I
Previous Lab Members

Tianrui Liu, BA
Research Intern
Undergraduate: University of Wisconsin-Madison

Manoj Kumar, MS, PhD
Research Assistant (Graduate Student)
UW Institute of Clinical and Translational Research Clinical Investigation Program
Undergraduate: Uttar Pradesh Technical University, India
Graduate School: University of Wisconsin–Madison, Master of Science in Biotechnology Program

Ciara Michel
Undergraduate Student
Undergraduate: University of Wisconsin–Madison, Microbiology
Graduate School: Emory University, Master of Public Health
Medical School: University of Wisconsin School of Medicine and Public Health

Kyle Kloepping, PhD
Research Associate (Post-Doc)
Undergraduate: University of Wisconsin–Platteville (Platteville, WI)
Graduate School: University of Iowa, Free Radical and Radiation Biology Program

Ginny Powers, PhD
Assistant Scientist
Undergraduate: St. Norbert College (De Pere, WI)
Graduate School: University of Wisconsin–Madison, Molecular and Cellular Pharmacology Program
Postdoctoral: University of Wisconsin–Madison, Pharmaceutical Sciences

Michael DeGrave, BS
2015 Shapiro Summer Research Scholar
University of Wisconsin School of Medicine and Public Health
Undergraduate: University of Wisconsin–Madison
Contact Information
Amy Fowler, MD, PhD
Principal Investigator and Associate Professor
Department of Radiology
University of Wisconsin School of Medicine and Public Health
600 Highland Avenue
Madison, WI 53792-3252
Contact: AFowler@uwhealth.org