
A UW Department of Radiology team of interventional radiologists, led by Erica Knavel Koepsel, MD, performed the first clinical histotripsy-based ablation in Wisconsin since the Food and Drug Administration (FDA) approved the procedure. Performed on January 11, 2024, it was also the first completed by a radiology department in the United States.
The procedure, approved by the FDA in October 2023, is a completely non-invasive, non-thermal approach to treating liver cancer and cancers that have metastasized to the liver. A specialized ultrasound treatment head delivers short, high amplitude pulses to targeted cancer cells, creating a bubble cloud that destroys the cells by expanding and contracting around them rapidly. Ultrasound imaging is used to monitor the formation and performance of the bubble cloud in real-time by the team.
The treatment zone and tissue treated by histotripsy is highly precise. Dr. Knavel Koepsel explained “Different tissues have different susceptibilities to histotripsy; bile ducts and blood vessels are less susceptible than the actual liver tissue, so this procedure avoids damaging other liver structures.”
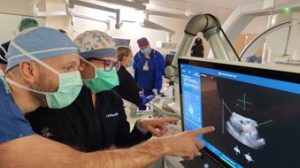
Unlike other treatment methods, histotripsy-based ablation requires only a single session, and patients will hopefully be able to return home the same day. Another exciting and unexpected benefit for patients was observed during clinical trials; treating one mass via histotripsy can have abscopal effects on other masses, causing them to reduce in size without receiving direct treatment.
Although the FDA’s approval only covers the treatment of liver cancer, the procedure could potentially treat other cancers. The department aims to conduct clinical trials using histotripsy to treat renal tumors.
Prior to this landmark case, the department played key roles in research and clinical trials required to gain FDA approval for histotripsy-based ablation.
Fred Lee, MD; Paul Laeseke, MD, PhD; and Timothy Ziemlewicz, MD were at the forefront throughout the process, including the development of the new technology needed for the procedure. In addition, they along with John Swietlik, MD dedicated over five years to conducting pre-clinical research. In addition, Dr. Ziemlewicz served as a principal investigator of the THERESA national clinical trial. Meghan Lubner, MD, an expert in locoregional therapies of the liver, served as a principal investigator for the #HOPE4LIVER site trial at UW-Madison.
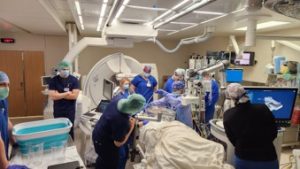
Dr. Knavel Koepsel anticipates the ablation team will incorporate histotripsy-based ablation as a mainstay option to treat liver cancer. “We’re really excited to bring this technology to UW and our patients to make a difference in cancer care by providing a non-invasive and highly precise treatment option, improving the tools we have available to treat liver cancer and hopefully, other cancers, in the future.”